Water Hardness Conversion
This is an invaluable resource for clients who require precise conversions between common units of water hardness. It translates measurements such as ppm, grains per gallon, and various degrees of hardness into the desired units. The underlying methodology and conversion factors are sourced from the authoritative work of Dr. Marco Wellinger, Dr. Samo Smrke, and Prof. Dr. Chahan Yeretzian in "The SCA Water Quality Handbook" (ISBN 978-0-9995807-3-8, page 16).
0
Please note the answer is an approximation and rounded to 2 significant figures.
Total Hardness Adjustment Calculator
Our Total Hardness Adjustment Calculator is designed to assist in accurately increasing the total hardness of water, specifically tailored for applications in the water treatment and brewing industries. This tool calculates the precise amounts of Calcium Chloride (CaCl₂) and Magnesium Chloride (MgCl₂) required to raise the total hardness of a given volume of water to a desired level.
0
Please note that these are approximate figures.
Calcium Hardness(Hᶜ) is the measure of Ca²⁺ ions in a solution as CaCO₃. It could also be viewed as a portion of total hardness(TH). It is best to view Hᶜ as a part of TH rather than an independent measure, because rarely does Ca²⁺ exist without Mg²⁺ in nature(Calcium and magnesium in drinking-water public health significance, 2009, p. 46). Water quality should never be viewed from a singular measure without factoring in other intertwining and interrelated constitutes. It is therefore suggested that Calcium Hardness is viewed within the context of its governing system. To truly understand what Hᶜ is, understanding Water Hardness and how to interpret it from a water analysis report is a fundamental principle that will not only aid in a better understanding of Calcium Hardness, but also explain Total Hardness, Alkalinity, Carbonate Hardness, and Non-Carbonate Hardness.
Despite various theories on the coining of the term “Hard Water”; whether it was an expansion on the previously used term “Harsh Water”(Sprat, et al., 1722, p. 292) or a yet to be concluded etymological mystery, we can astutely infer with a certain degree of certainty that the term was originally derived from the effects rather than the cause. The simplest experiment one could conduct to assess the general hardness of water and reach a similar generalized conclusion that led to the coining of the term is to work up a lather in a sodium salt based soap(control & test samples); using bottled water and comparing the exerted work and agitation time to reach an adequate amount of foam with a known Hard Water source. Although it is not an accurate or scientific experiment, it does shed light on an unusual characteristic on the effects of water on soap. One might note that “Hard Water” will take much longer to produce a sufficient amount of foam when compared with “Soft Water”, or require more soap to reach the same result as that of Soft Water’s precipitation of soap. Extrapolating from the soap experiment, water hardness could be viewed and defined in terms of its effect as the capacity of soap precipitation(Ambasta, 2008, p. 79). In a Sodium Stearate(A long-chain fatty acid salt(Wist, Lehr, & McEachern, 2009, p. 34)) based soap, Calcium and Magnesium ions in “Hard Water” will react with the surfactant anion to produce the build up of “Soap Scum/Soap Curd”; the remains of insoluble calcium and/or magnesium salt. Water hardness from this perspective is defined as the concentration of calcium(Ca²⁺ ) and magnesium(Mg²⁺) ions in a water solution(Wist, Lehr, & McEachern, 2009, p. 34). A more generalized and formal definition encompasses all alkaline earth metals as well as other common metals found in natural water. Although in practice, all multivalent cations--other than Calcium and Magnesium--are disregarded due to their commonly insignificant amounts in freshwater, it is therefore an approximate measure to view Hardness in terms of the sum of Calcium and Magnesium.
According to the American Water Works Association(Water Treatment, 2003, p. 315, 316), the specific inclusion of Calcium Hardness(Hᶜ) and Magnesium Hardness(Hᵐ) as accurate approximative drivers behind Total Hardness(TH) is the result of what is normally present in freshwater in terms of trace amounts. Total Hardness(TH) could therefore be expressed as:
TH = Hᶜ + Hᵐ
Where:
Hᶜ is Ca²⁺ measured in mg/l as CaCO₃§
Hᵐ is Mg²⁺ measured in mg/l as CaCO₃§
Total Hardness(TH) is also expressed in terms carbonate hardness(CH) and non-carbonate hardness(NCH):
TH = CH + NCH
Where:
CH is measured in mg/l as CaCO₃§
NCH is measured in mg/l as CaCO₃§
§ CaCO3(Calcium Carbonate) is a common unit of expression for hardness concentration.
Carbonate hardness(CH) is often called temporary hardness because it can be removed or precipitated by heat. Heat releases carbon dioxide(CO₂) from the solution by decomposing the bicarbonate salts of calcium(Ca(HCO₃)₂) and magnesium(Mg(HCO₃)₂)(Davis, "7", 2017, p. 3):
Ca²⁺ + 2HCO⁻₃ ⇌ CaCO₃ + CO₂ + H₂O
Where:
HCO⁻₃ is hydrogen carbonate--bicarbonate(anion)
CaCO₃ is a precipitate as a solid
CO₂ is a gas
Non-carbonate hardness(NCH) is often called permanent hardness because heat does not remove nor does it precipitate the non-carbonate salts. These salts are Calcium and Magnesium salts other than bicarbonate salts. The most common non-carbonate salts are: Calcium Sulfate(CaSO₄), Calcium Chloride(CaCl₂), Magnesium Sulfate(MgSO₄), and Magnesium Chloride(MgCl₂)(Water Treatment, 2003, p. 315, 316).
Carbonate Hardness(CH ) has a direct relationship with alkalinity; it is defined as the concentration of hardness amounting to the total hardness or the total alkalinity, whichever is less(Davis, "7", 2017, p. 3). Whereas Non-carbonate hardness(NCH) is defined in terms of total hardness in excess of total alkalinity. To better explain this point, Example 1 is put forth.
Example 1; adapted from Davis -- Assume the following water analysis results-Table 1:
This is a fictitious report, the values were selected as such to reach a balance in equivalents(meq/l). It is common to find a ±5% discrepancy between the totals of cations and anions. If the charge balance error(CBE) was greater than 5%, it would either suggest that there were ions not measured for or there was an error during the testing process.
Before calculating TH the conversion of compounds from mg/l as ions to mg/l as CaCO₃ is required. The equivalent weight(EW) of an ion(Not necessarily an ion; the correct chemical term is Chemical Species which include ions, atoms, and molecules among others) is the quotient of the ion’s molecular weight(MW) and its valency(V):
EW = MW/V
To convert from mg/l as ion to mg/l as CaCO₃:
mg/l as CaCO₃ = (mg/l as ion) * E
Where:
E = (EW(CaCO3)/EW(Ion))
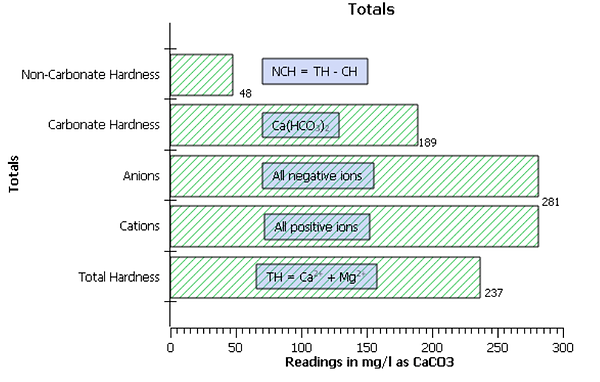
The readings can be rewritten--Table 2--in terms of mg/l as CaCO₃ as follows--Bar Chart 1 for a visual representation:
Bar Chart 1
Cations total to 281 mg/l as CaCO₃; with hardness representing 237 mg/l as CaCO₃. Total Hardness was determined by utilizing Equation 1 as follows:
TH = Ca²⁺ + Mg²⁺
= 217 + 20 = 237 mg/l as CaCO₃
Anions total to 281 mg/l as CaCO₃; with carbonate hardness equating to 189 mg/l as CaCO₃. Carbonate hardness was determined as HCO⁻₃ = 189 mg/l as CaCO₃ because it is less than TH. As stated earlier, Carbonate hardness equals the total hardness or the total alkalinity, whichever is less. Since TH is greater than total alkalinity, carbonate hardness equals total alkalinity.
Alkalinity = [HCO⁻₃ ] + 2[CO₃²⁻] + [OH⁻]-[H⁺]
However, it is unlikely that the water to be used will have a pH lower than 6 or greater than 8, therefore, Hydrogen(H⁺) and Hydroxide(OH⁻) ions are to be disregarded:
Alkalinity = [HCO⁻₃ ] + 2[CO₃²⁻]
Since our analysis does not contain carbonate(CO₃²⁻), alkalinity will equal bicarbonate(HCO⁻₃) = 189 mg/l as CaCO₃. Non-carbonate hardness totals to 48 mg/l as CaCO₃ using Equation 2 as follows:
TH = CH + NCH
NCH = TH - CH
NCH = 237 - 189 = 48 mg/l as CaCO₃
Bar Chart Representing Totals
In summary, water hardness is a measure of the concentration of certain minerals dissolved in water, primarily calcium and magnesium ions. These minerals are typically absorbed by water as it moves through soil and rock. The hardness of water is usually quantified in grains per gallon (gpg), parts per million (ppm), or milligrams per liter (mg/L), and it's classified in degrees of hardness based on the scale of mineral content.
Here’s a brief explanation of the different levels of water hardness:
-
Soft Water: Typically contains less than 17.1 ppm or 1 gpg of calcium carbonate. Soft water is generally free from the minerals that cause hardness.
-
Slightly Hard: Measures between 17.1 ppm and 60 ppm or 1 to 3.5 gpg.
-
Moderately Hard: The water hardness level is between 61 ppm and 120 ppm, or 3.6 to 7 gpg.
-
Hard: Falls within the range of 121 ppm to 180 ppm, or 7.1 to 10.5 gpg.
-
Very Hard: Any reading above 180 ppm or over 10.5 gpg is considered very hard.
Hard water can lead to various issues in household and industrial settings, such as:
-
Scaling: The minerals can deposit on the surfaces of pipes, boilers, and cookware, forming a hard, chalky buildup called scale, which can reduce the efficiency of heating elements and clog pipes.
-
Soap Scum: Hard water reacts with soap to form a sticky residue known as soap scum, which can be difficult to clean and can leave deposits on fixtures, tiles, and fabrics.
-
Dingy Laundry: Clothes washed in hard water may look dull and feel rough, as the minerals interfere with the cleaning effectiveness of laundry detergents.
-
Skin and Hair Issues: Bathing in hard water can leave skin feeling dry and itchy due to soap residue, and it can make hair look dull and feel sticky.
Water softening systems or conditioners are commonly used to reduce water hardness by removing or altering the minerals responsible for the hardness, providing water that is more compatible with soap, extending the lifespan of plumbing, and improving the effectiveness of water heaters and other appliances.
So, why would we want to increase total hardness?
According to The SCA Water Quality Handbook(Dr. Marco Wellinger, Dr. Samo Smrke, and Prof. Dr. Chahan Yeretzian in "The SCA Water Quality Handbook".), optimal water composition significantly impacts the sensory quality of coffee. Total hardness is believed to improve extraction efficiency, affecting flavor balance and aroma, though laboratory measurements with a coffee refractometer have not confirmed this. Conversely, alkalinity is known to reduce perceived acidity, and high levels can lead to over-extraction due to the formation of carbon dioxide. Recommendations for optimal water hardness and alkalinity for coffee extraction vary, with the range for hardness being broader (51-175 ppm CaCO₃) compared to a narrower range for alkalinity (40-75 ppm CaCO₃). These guidelines are mainly for espresso but are also relevant for brewed coffee. For cupping, softer water is sometimes preferred. It's important to avoid very low alkalinity in espresso machines to prevent corrosion. The SCA 'Core Zone' provides a balanced guideline for espresso machines and brew boilers, considering both technical and sensory aspects to ensure safe operation and high-quality brews.
REFERENCES
Ambasta, B. K., (2008). In Chemistry for engineers. Laxmi Publications.
World Health Organization. (2009). Calcium and magnesium in drinking-water public health significance. Geneva (Switzerland).
Davis, M. L. (2017). 7. In Water and Wastewater Engineering: Design Principles and Practice. McGraw-Hill.
Sprat, T., Morice, W., Hooke, R., Knapton, J., Walthoe, J., Tooke, B., (1722). In The History of the Royal Society of London for the Improving of Natural Knowledge. 2. Ed. London: Printed for J. Knapton, J. Walthoe, B. and S. Tooke, D. Midwinter, B. Cowse, J. Tonson, R. Robinson, J. Wilford, and S. Chapman.
American Water Works Association. (2003). In Water Treatment.
Watson, D. G. (2011). In Pharmaceutical Chemistry E-Book. Churchill Livingstone.
Wist, W., Lehr, J. H., & McEachern, R. J. (2009). In Water softening with potassium chloride: process, health, and environmental benefits. Hoboken: Wiley.